-
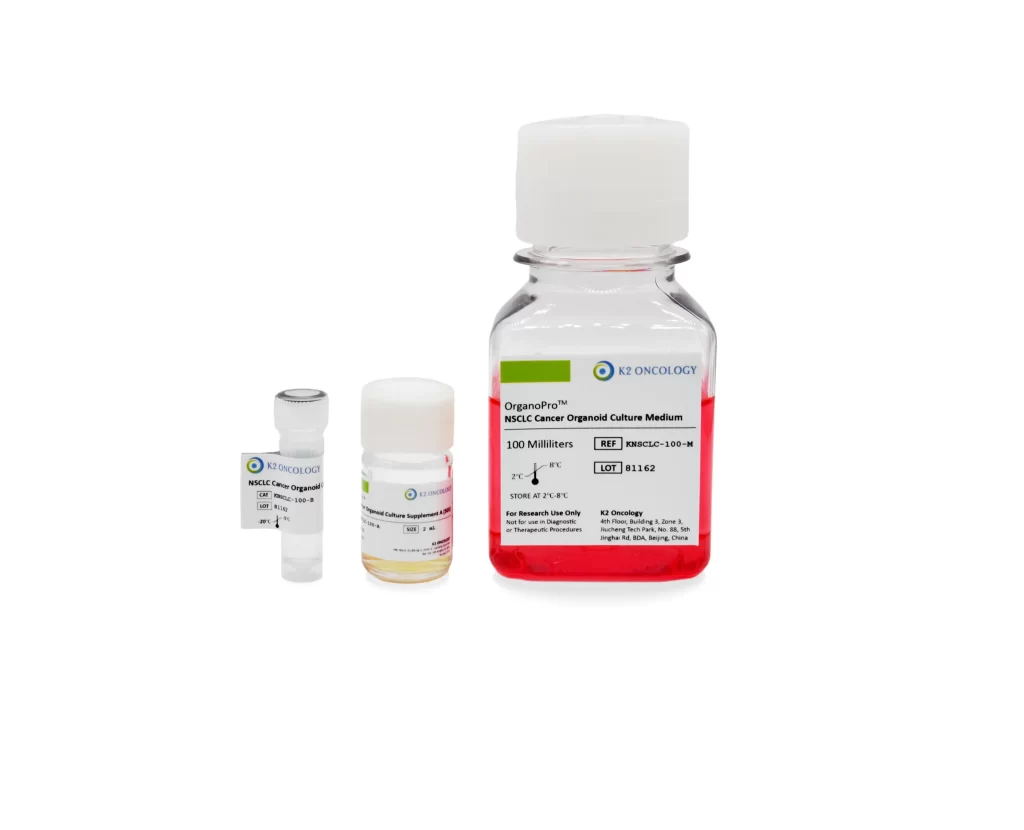
非小细胞肺癌类器官培养试剂盒
KNSCLC-100
-

非小细胞肺癌类器官培养试剂盒
KNSCLC-1000


OrganoPro™非小细胞肺癌类器官培养基套装
KNSCLC-100 包含以下产品
- OrganoPro™非小细胞肺癌培养基 100mL
- OrganoPro™非小细胞肺癌添加剂成分A 2mL
- OrganoPro™非小细胞肺癌添加剂成分B 1mL
KNSCLC-1000 包含以下产品
- OrganoPro™非小细胞肺癌培养基 1000mL
- OrganoPro™非小细胞肺癌添加剂成分A 10mL x 2
- OrganoPro™非小细胞肺癌添加剂成分B 10mL

我们的科学家向您推荐
我们的产品简化了实验流程,集成多种因子,无需单独优化,扩增潜力高,14天内细胞数量可达到1×10^6。适用于多种培养形式,包括基质胶、低吸附孔板和生物反应器悬浮培养。GMP级别生产条件下制备,批次质量稳定,试剂含量是常规市售干细胞培养基的2倍,实现极佳的成本效益比。让复杂的培养变得简单快速,让科研变得更高效。
概览
此产品基于 Simumatrix 技术平台,通过工业化高通量筛选,针对中国高发的肿瘤类型进行培养基优化筛选而开发出的类器官培养基产品,可用于非小细胞肺癌的类器官培养。
产品优势/特点:
- 简单易用,节省验证时间:提供详细操作方案,产品使用简便,节省研究者大量类器官培养摸索验证时间;
- 肿瘤组织覆盖类型广:覆盖多达15个组织瘤种,>900种肿瘤驱动基因突变模型;
- 扩增潜力高:自研高活力高稳定性WNT与RSPOs,支持肿瘤类器官多代次连续稳定培养;
- 肿瘤类器官验证数据齐备:多维类器官验证数据的整合,类器官驱动基因突变及表达谱,类器官组织病理学验证及类器官药敏数据等。
- 多篇高分文献应用:多篇高分文献应用,口碑卓越;
- 自主研发,产能充足,性价比高:全自研生产,源头品控,产品性价比高。
产品组成:
| 产品名称 | 货号 | 规格 | 储存温度 | 保质期 |
|---|---|---|---|---|
| OrganoPro™ NSCLC Cancer Organoid Culture Medium 人源非小细胞肺癌类器官培养基 | KNSCLC-100/1000-M | 100mL / 1000mL | 2-8°C | 12个月 |
| OrganoPro™ NSCLC Cancer Organoid Culture Supplement A(50X) 人源非小细胞肺癌类器官培养基添加剂A(50X) | KNSCLC-100/1000-A | 2mL / 20mL | -20°C | 12个月 |
| OrganoPro™ NSCLC Cancer Organoid Culture Supplement B(100X) 人源非小细胞肺癌类器官培养基添加剂B(100X) | KNSCLC-100/1000-B | 1mL / 10mL | -20°C | 12个月 |
类型
类器官培养基
适用细胞
非小细胞肺癌类器官
物种
人类
应用
培养人源非小细胞肺癌类器官
商标
OrganoPro™
产品使用说明及支持信息
在产品文档中查找支持信息和使用说明,或在下方探索更多
| 文档类型 | 产品名称 | Catalog # |
|---|---|---|
| User manual | OrganoPro™非小细胞肺癌类器官培养基套装 | KNSCLC-100 KNSCLC-1000 |
资源及文献引用
相关资源及文献引用
PRMT3 Drives IDO1-Dependent Radioresistance and Immunosuppression by Promoting Kynurenine Metabolism in Non-Small Cell Lung Cancer
Shijie Zhang, et al. | Cancer Res. (2025) Oct 23.
ABSTRACT:
Inhibition of CPT1A activates the cGAS/STING pathway to enhance neutrophil-mediated tumor abrogation in triple-negative breast cancer
Chenglong Li, et al. | Nat Commun. (2025) Jul 28;16 (1): 6929.
ABSTRACT:
Nuclear to Cytoplasmic Transport Is a Druggable Dependency in HDAC7-driven Small Cell Lung Cancer
Tingting Qin, et al. | Advanced Science (2025)
ABSTRACT:
Organoid drug screening report for a non-small cell lung cancer patient with EGFR gene mutation negativity: A case report and review of the literature
Pan, Yuetian, Hongshang Cui, and Yongbin Song | Frontiers in Oncology (2023)
Abstract:
Identification of solamargine as a cisplatin sensitizer through phenotypical screening in cisplatin-resistant NSCLC organoids
Han, Yi,et al. | Frontiers in Pharmacology (2022)
Abstract:
An Artemisinin Derivative ART1 Induces Ferroptosis by Targeting the HSD17B4 Protein Essential for Lipid Metabolism and Directly Inducing Lipid Peroxidation.
Xie, Jingjing, et al. | CCS Chemistry (2022)
Abstract:
Artemisinin and its derivatives, commonly known as antimalarial drugs, have gradually come to be regarded as potential antitumor agents, although their cytotoxic efficacy and mechanisms of action remain to be settled. Herein, we report that an artemisinin analog, ART1, can potently induce ferroptosis in a subset of cancer cell lines. Structure–activity relationship (SAR) analysis reveals that both the endoperoxide moiety and the artemisinin skeleton are required for the antitumor activity of ART1. Aided with ART1-based small-molecule tools, chemical proteomic analysis identified the HSD17B4 protein as a direct target of ART1. HSD17B4 resides in peroxisomes and is an essential enzyme in the catabolism of very-long-chain fatty acids. Our results demonstrate that ART1 initiates ferroptosis through selective oxidation of the fatty acids in peroxisomes by hijacking the HSD17B4 protein without disturbing its enzymatic function, providing a promising mechanism to develop therapeutics for cancer treatment. Read More: https://doi.org/10.31635/ccschem.021.202000691Pyrotinib in patients with HER2-amplified advanced non–small cell lung cancer: A prospective, multicenter, single-arm trial
Song, Zhengbo,et al. | Clinical Cancer Research (2022)
Abstract:
Glutamine synthetase licenses APC/C-mediated mitotic progression to drive cell growth
Zhao, Jiang-Sha,et al. | Nature Metabolism (2022)
Abstract:
Halofuginone sensitizes lung cancer organoids to cisplatin via suppressing PI3K/AKT and MAPK signaling pathways
Li, Hefei,et al. | Frontiers in Cell and Developmental Biology (2021)
Abstract:
Lung cancer is the leading cause of cancer death worldwide. Cisplatin is the major DNA-damaging anticancer drug that cross-links the DNA in cancer cells, but many patients inevitably develop resistance with treatment. Identification of a cisplatin sensitizer might postpone or even reverse the development of cisplatin resistance. Halofuginone (HF), a natural small molecule isolated from Dichroa febrifuga, has been found to play an antitumor role. In this study, we found that HF inhibited the proliferation, induced G0/G1 phase arrest, and promoted apoptosis in lung cancer cells in a dose-dependent manner. To explore the underlying mechanism of this antitumor effect of halofuginone, we performed RNA sequencing to profile transcriptomes of NSCLC cells treated with or without halofuginone. Gene expression profiling and KEGG analysis indicated that PI3K/AKT and MAPK signaling pathways were top-ranked pathways affected by halofuginone. Moreover, combination of cisplatin and HF revealed that HF could sensitize the cisplatin-resistant patient-derived lung cancer organoids and lung cancer cells to cisplatin treatment. Taken together, this study identified HF as a cisplatin sensitizer and a dual pathway inhibitor, which might provide a new strategy to improve prognosis of patients with cisplatin-resistant lung cancer.
COA查询
根据货号和批次号,在线查询已购买产品的COA证书
产品货号和产品批号均显示在产品标签上对应位置(如右侧示意图所示)
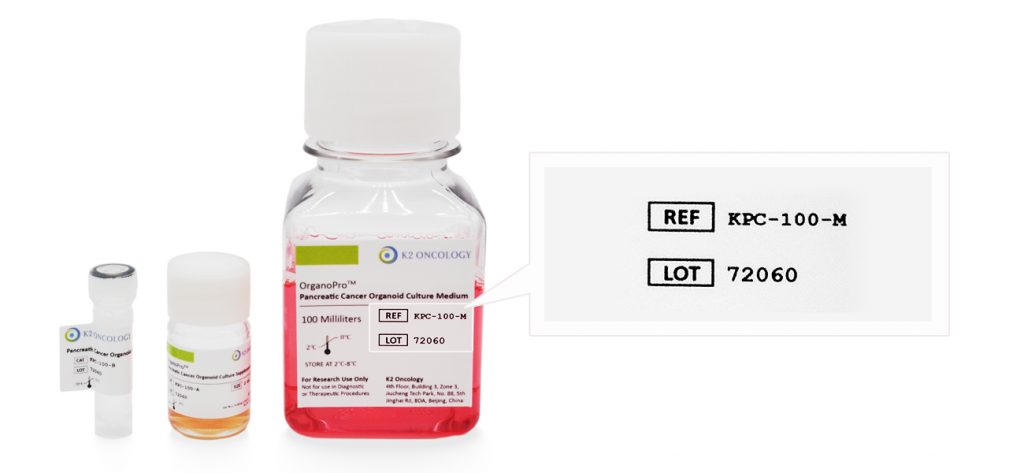
Ref/Cat#
产品货号,每个产品对应的独立货号
Lot#
批次编号,同一产品不同批次会有不同批号,请您在产品包装找到对应批号