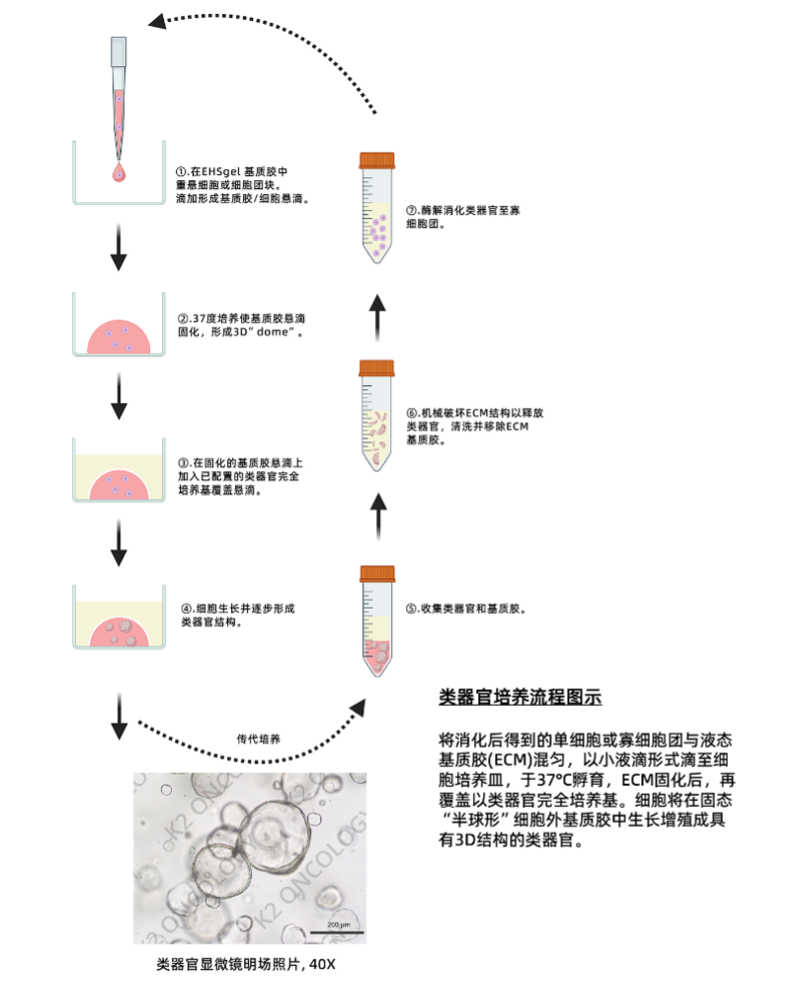
-
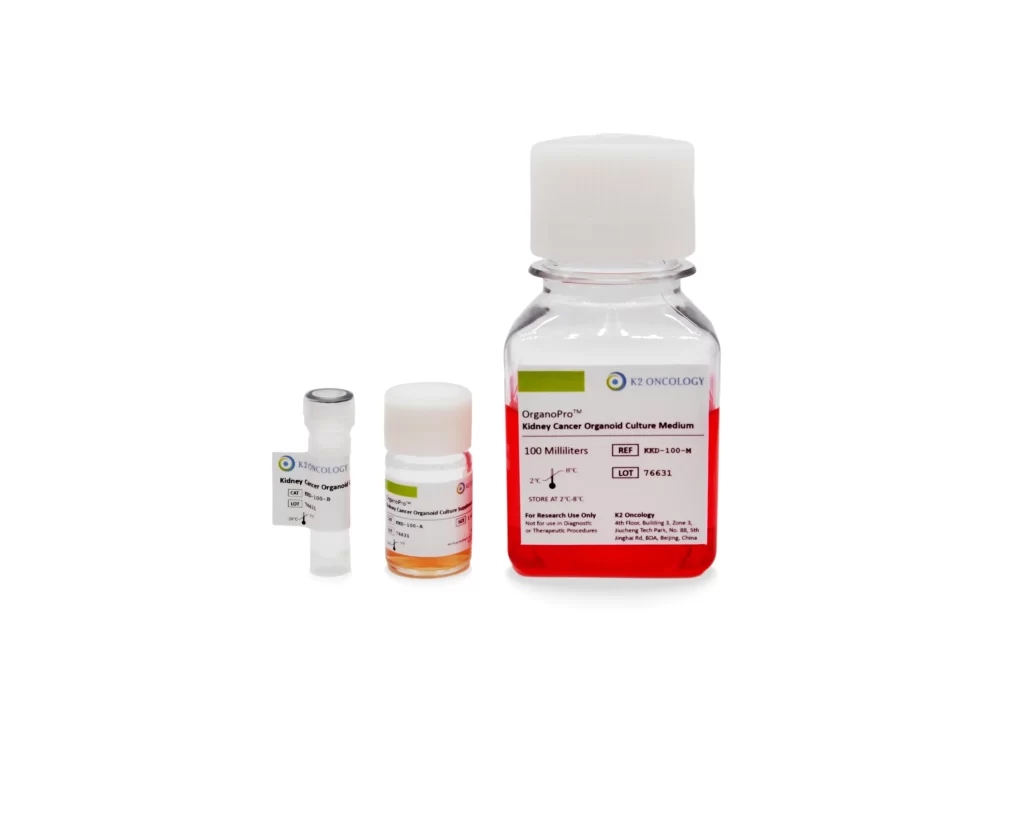
肾癌类器官培养试剂盒
KKD-100
-

肾癌类器官培养试剂盒
KKD-1000
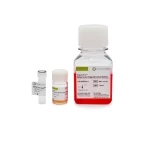

OrganoPro™肾癌类器官培养基套装
KKD-100 包含以下产品
- OrganoPro™肾癌培养基 100mL
- OrganoPro™肾癌添加剂成分A 2mL
- OrganoPro™肾癌添加剂成分B 1mL
KKD-1000 包含以下产品
- OrganoPro™肾癌培养基 1000mL
- OrganoPro™肾癌添加剂成分A 10mL x 2
- OrganoPro™肾癌添加剂成分B 10mL
科途医学 科学家

名字
PhD
我们的科学家向您推荐
我们的产品简化了实验流程,集成多种因子,无需单独优化,扩增潜力高,14天内细胞数量可达到1×10^6。适用于多种培养形式,包括基质胶、低吸附孔板和生物反应器悬浮培养。GMP级别生产条件下制备,批次质量稳定,试剂含量是常规市售干细胞培养基的2倍,实现极佳的成本效益比。让复杂的培养变得简单快速,让科研变得更高效。
概览
此产品基于 Simumatrix 技术平台,通过工业化高通量筛选,针对中国高发的肿瘤类型进行培养基优化筛选而开发出的类器官培养基产品,可用于肾癌的类器官培养。
产品优势/特点:
- 简单易用,节省验证时间:提供详细操作方案,产品使用简便,节省研究者大量类器官培养摸索验证时间;
- 肿瘤组织覆盖类型广:覆盖多达15个组织瘤种,>900种肿瘤驱动基因突变模型;
- 扩增潜力高:自研高活力高稳定性WNT与RSPOs,支持肿瘤类器官多代次连续稳定培养;
- 肿瘤类器官验证数据齐备:多维类器官验证数据的整合,类器官驱动基因突变及表达谱,类器官组织病理学验证及类器官药敏数据等。
- 多篇高分文献应用:多篇高分文献应用,口碑卓越;
- 自主研发,产能充足,性价比高:全自研生产,源头品控,产品性价比高。
产品组成:
| 产品名称 | 货号 | 规格 | 储存温度 | 保质期 |
|---|---|---|---|---|
| OrganoPro™ Kideny Cancer Organoid Culture Medium 肾癌类器官培养基 | KKD-100/1000-M | 100mL / 1000mL | 2-8°C | 12个月 |
| OrganoPro™ Kideny Cancer Organoid Culture Supplement A(50X) 肾癌类器官培养基添加剂A(50X) | KKD-100/1000-A | 2mL / 20mL | -20°C | 12个月 |
| OrganoPro™ Kideny Cancer Organoid Culture Supplement B(100X) 肾癌类器官培养基添加剂B(100X) | KKD-100/1000-B | 1mL / 10mL | -20°C | 12个月 |
类型
类器官培养基
适用细胞
人源肾癌类器官
物种
人类
应用
培养肾癌类器官
商标
OrganoPro™
产品使用说明及支持信息
在产品文档中查找支持信息和使用说明,或在下方探索更多
| 文档类型 | 产品名称 | Catalog # |
|---|---|---|
| User manual | OrganoPro™肾癌类器官培养基套装 | KKD-100 KKD-1000 |
资源及文献引用
相关资源及文献引用
Phenotypical screening on metastatic PRCC-TFE3 fusion translocation renal cell carcinoma organoids reveals potential therapeutic agents
Cao, Chuanzhen, et al. | Clinical and Translational Oncology (2022)
Abstract:
Purpose
Translocation renal cell carcinoma (tRCC) is a subtype that occurs predominantly in children and young individuals. Metastatic tRCC occurring in young patients is more aggressive than that occurring in older patients, and there are still no effective therapies. Organoids can mimic original tissues and be assessed by high-throughput screening (HTS). We aimed to utilize patient-derived organoids and HTS to screen drugs that can be repurposed for metastatic tRCC with PRCC-TFE3 fusion.
Methods
Tumor tissues were obtained from treatment-naïve metastatic tRCC patients who underwent surgery. Histopathology and fluorescence in situ hybridization (FISH) confirmed the tRCC. Organoids derived from the dissected tissues were cultured and verified by FISH and RNA-seq. HTS was performed to seek promising drugs, and potential mechanisms were explored by RNA-seq and cell-based studies.
Results
We successfully established a metastatic tRCC organoid with PRCC-TFE3 fusion, a common fusion subtype, and its characteristics were verified by histopathology, FISH, and RNA-seq. An HTS assay was developed, and the robustness was confirmed. A compound library of 1816 drugs was screened. Eventually, axitinib, crizotinib, and JQ-1 were selected for further validation and were found to induce cell cycle arrest and apoptosis. RNA-seq analyses of posttreatment organoids indicated that crizotinib induced significant changes in autophagy-related genes, consistent with the potential pathogenesis of tRCC. Conclusions We established and validated organoids derived from tissues dissected from a patient with metastatic tRCC with PRCC-TFE3 fusion and achieved the HTS process for the first time. Crizotinib might be a targeted therapy worthy of exploration in the clinic for metastatic tRCC with PRCC-TFE3 fusion. Such organoid and HTS assays may represent a promising model system in translational research assisting in the development of clinical strategies.
Read More: https://doi.org/10.1007%2Fs12094-021-02774-8 Translocation renal cell carcinoma (tRCC) is a subtype that occurs predominantly in children and young individuals. Metastatic tRCC occurring in young patients is more aggressive than that occurring in older patients, and there are still no effective therapies. Organoids can mimic original tissues and be assessed by high-throughput screening (HTS). We aimed to utilize patient-derived organoids and HTS to screen drugs that can be repurposed for metastatic tRCC with PRCC-TFE3 fusion.
Methods
Tumor tissues were obtained from treatment-naïve metastatic tRCC patients who underwent surgery. Histopathology and fluorescence in situ hybridization (FISH) confirmed the tRCC. Organoids derived from the dissected tissues were cultured and verified by FISH and RNA-seq. HTS was performed to seek promising drugs, and potential mechanisms were explored by RNA-seq and cell-based studies.
Results
We successfully established a metastatic tRCC organoid with PRCC-TFE3 fusion, a common fusion subtype, and its characteristics were verified by histopathology, FISH, and RNA-seq. An HTS assay was developed, and the robustness was confirmed. A compound library of 1816 drugs was screened. Eventually, axitinib, crizotinib, and JQ-1 were selected for further validation and were found to induce cell cycle arrest and apoptosis. RNA-seq analyses of posttreatment organoids indicated that crizotinib induced significant changes in autophagy-related genes, consistent with the potential pathogenesis of tRCC. Conclusions We established and validated organoids derived from tissues dissected from a patient with metastatic tRCC with PRCC-TFE3 fusion and achieved the HTS process for the first time. Crizotinib might be a targeted therapy worthy of exploration in the clinic for metastatic tRCC with PRCC-TFE3 fusion. Such organoid and HTS assays may represent a promising model system in translational research assisting in the development of clinical strategies.
暂无文献引用
COA查询
根据货号和批次号,在线查询已购买产品的COA证书
请输入产品货号(REF or CAT Number) 和产品批号(LOT Number) ,查询COA证书文件。
产品货号和产品批号均显示在产品标签上对应位置(如右侧示意图所示)
产品货号和产品批号均显示在产品标签上对应位置(如右侧示意图所示)
产品货号
﹡
产品批号
﹡
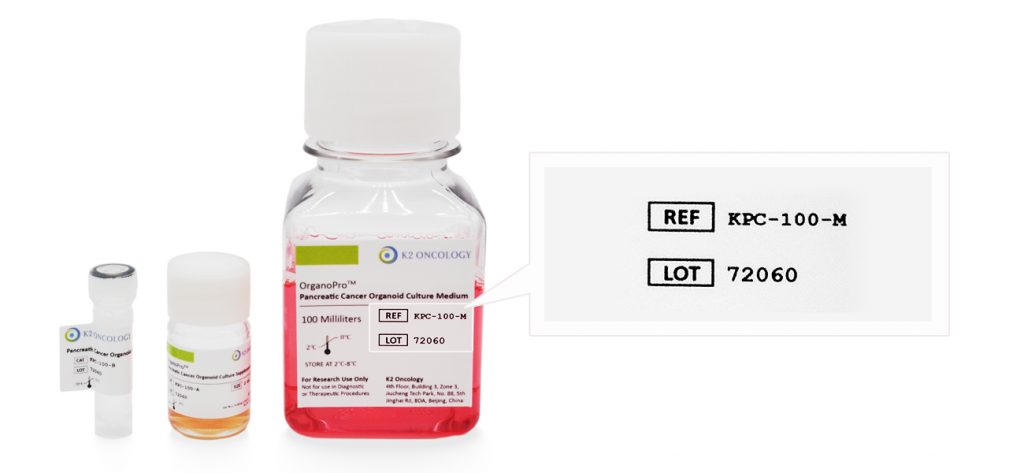
Ref/Cat#
产品货号,每个产品对应的独立货号
Lot#
批次编号,同一产品不同批次会有不同批号,请您在产品包装找到对应批号